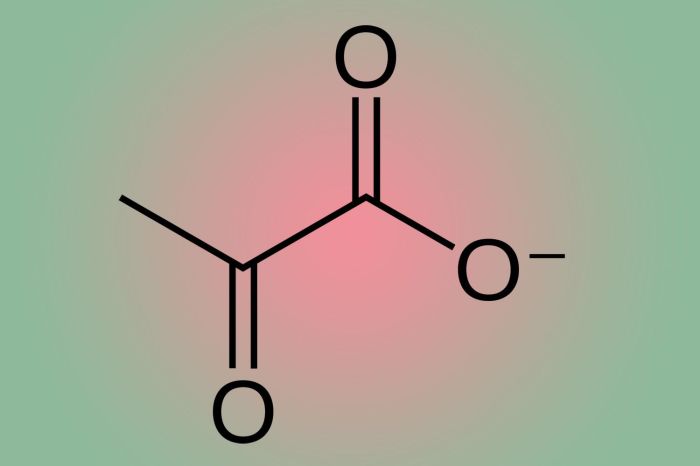
Pyruvate structure at ph 7.4 – At the heart of biological processes, pyruvate stands as a pivotal molecule. This article embarks on an exploration of its intricate structure at pH 7.4, delving into its chemical makeup, molecular characteristics, and biological significance.
Pyruvate, an organic acid, possesses a molecular formula of C3H4O3 and a molar mass of 88.06 g/mol. Its molecular structure features a carboxyl group (-COOH) and a keto group (-CO-), imparting both acidic and carbonyl properties.
Pyruvate Structure
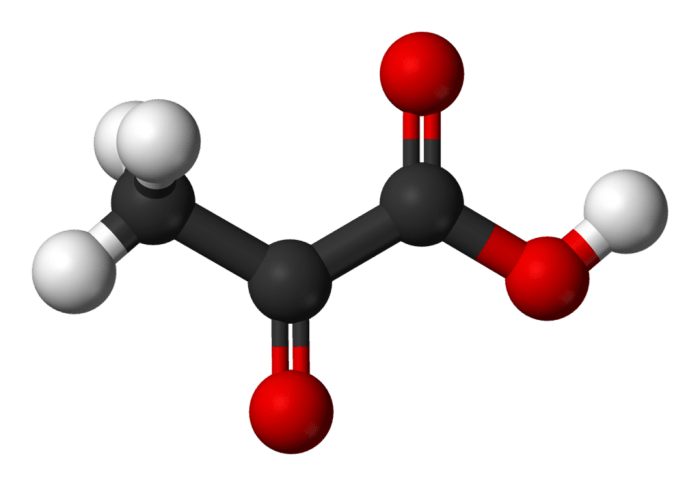
Pyruvate is a three-carbon organic acid that plays a crucial role in cellular respiration, the process by which cells convert glucose into energy. It is the product of glycolysis, the first stage of cellular respiration, and serves as a substrate for the citric acid cycle, the second stage of cellular respiration.
Chemical Structure
The chemical structure of pyruvate consists of a carboxyl group (-COOH) attached to a two-carbon keto group (-CO-CH3). The carboxyl group is located at the end of the molecule, while the keto group is located in the middle. The molecular formula of pyruvate is C3H4O3, and its molar mass is 88.06 g/mol.
Molecular Illustration
The following illustration shows the molecular structure of pyruvate:
O || -C-C-C-OH || O
The carboxyl group is shown on the left, and the keto group is shown in the middle. The hydrogen atoms are not shown.
Pyruvate at pH 7.4
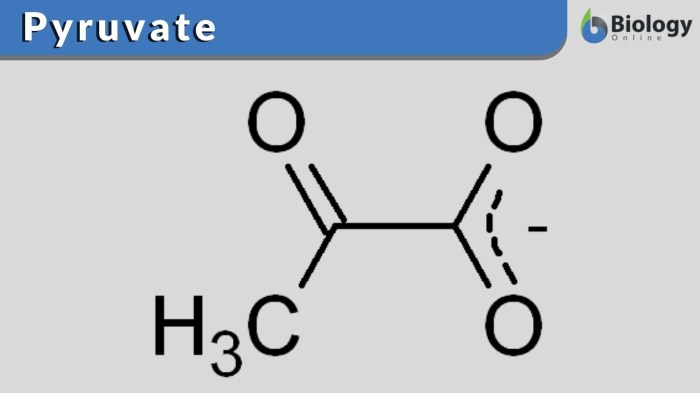
At pH 7.4, the predominant form of pyruvate is the enolate anion. This is due to the fact that the pKa of the enol group in pyruvate is 2.5, which means that at pH 7.4, the majority of the pyruvate molecules will exist in the enolate form.
Acid Dissociation Constants (pKa), Pyruvate structure at ph 7.4
The acid dissociation constant (pKa) is a measure of the strength of an acid. It is defined as the negative logarithm of the equilibrium constant for the dissociation of the acid. The lower the pKa, the stronger the acid.
The pKa of the enol group in pyruvate is 2.5, which means that it is a relatively weak acid. This is because the enol group is stabilized by resonance.
Pyruvate Species at pH 7.4
The following table summarizes the pKa values and corresponding pyruvate species at pH 7.4:
| pKa | Pyruvate Species |
|---|---|
| 2.5 | Enol |
| 9.2 | Keto |
Keto-Enol Tautomerism
Pyruvate exists in two isomeric forms: the keto form and the enol form. Keto-enol tautomerism is the reversible isomerization between these two forms, which occurs via the transfer of a proton from the α-carbon to the carbonyl oxygen.
The equilibrium between the keto and enol forms is influenced by several factors, including pH, temperature, and the presence of catalysts. At physiological pH (7.4), the keto form predominates, accounting for approximately 99.9% of the total pyruvate pool.
Factors Influencing Keto-Enol Equilibrium
- pH:The keto form is favored at low pH, while the enol form is favored at high pH.
- Temperature:The enol form is favored at higher temperatures.
- Catalysts:Enzymes, such as enolase, can catalyze the conversion between the keto and enol forms.
The keto-enol equilibrium is important in biological systems because the enol form is more reactive than the keto form. This allows pyruvate to participate in a variety of enzymatic reactions, including glycolysis, gluconeogenesis, and the citric acid cycle.
Pyruvate exists as a mixture of two forms at pH 7.4: the keto form and the enol form. The keto form is more stable than the enol form, but the enol form is more reactive. This is because the enol form has a double bond, which makes it more susceptible to attack by nucleophiles.
To enhance your understanding of organic chemistry concepts, consider exploring Wordly Wise 3000 Book 8 . This resource provides comprehensive coverage of the keto-enol equilibrium and other important organic chemistry topics.
Illustration of Keto-Enol Equilibrium
The following illustration depicts the keto-enol equilibrium of pyruvate at pH 7.4:

As shown in the illustration, the keto form (left) predominates, while the enol form (right) is a minor species.
Biological Significance
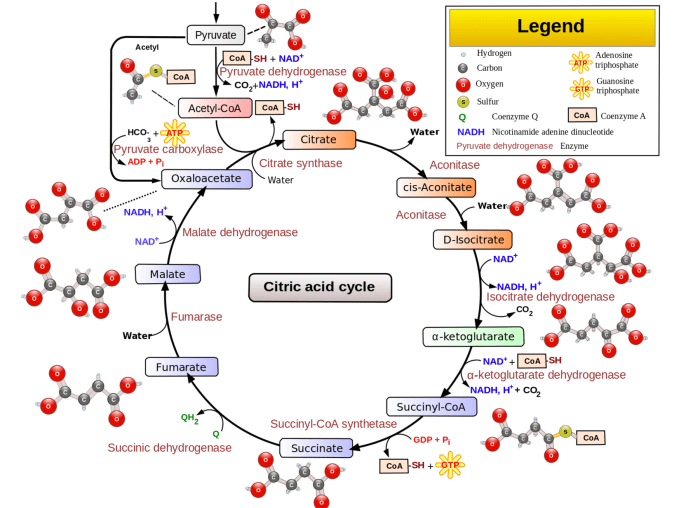
Pyruvate, a crucial molecule in cellular metabolism, plays a central role in energy production and various metabolic pathways. It is the end product of glycolysis, the initial stage of cellular respiration, where glucose is broken down to generate energy.Pyruvate is metabolized through different pathways depending on the cellular needs and availability of oxygen.
Under aerobic conditions, pyruvate enters the citric acid cycle (Krebs cycle), where it is further oxidized to produce carbon dioxide, water, and a significant amount of energy in the form of ATP (adenosine triphosphate).In the absence of oxygen, pyruvate undergoes anaerobic fermentation.
In this process, pyruvate is converted to lactate in animals, while in plants and microorganisms, it is converted to ethanol or other fermentation products. These fermentation pathways generate a smaller amount of energy compared to the citric acid cycle but allow cells to continue producing ATP under oxygen-limiting conditions.Pyruvate
is also a precursor for gluconeogenesis, the process of synthesizing glucose from non-carbohydrate sources such as lactate, amino acids, and fatty acids. This pathway is essential for maintaining blood glucose levels during periods of fasting or when glucose availability is limited.Enzymes
involved in pyruvate metabolism include:
- Pyruvate dehydrogenase: Converts pyruvate to acetyl-CoA for entry into the citric acid cycle.
- Lactate dehydrogenase: Converts pyruvate to lactate under anaerobic conditions.
- Pyruvate carboxylase: Converts pyruvate to oxaloacetate, the starting molecule for gluconeogenesis.
User Queries: Pyruvate Structure At Ph 7.4
What is the predominant form of pyruvate at pH 7.4?
The predominant form of pyruvate at pH 7.4 is the keto form.
How does pyruvate participate in cellular respiration?
Pyruvate is a key intermediate in cellular respiration, where it enters the citric acid cycle to generate energy.